Recently, to control supercooling of a phase change material (PCM), we are focusing on the solution structure of a supercooled aqueous solution.

BaSO4 nano-wire generated in the micro-emulsion
Japanese page / English page
| News | |
|---|---|
|
Assistant Prof. Sugahara joined in the Energy and Photochemical Engineering Gr. (Prof. Hirai and Associate Prof. Shiraishi Gr.) from April 2020.
|
| We have been studying so-called molecular clusters in the liquid solutions by use of scanning electron microscopy developed as "plasma replica SEM method", which combines SEM observation with the freeze-fracture replica method. The molecular cluster plays an important role of initiator of phase transition such as liquid-to-solid and gas-to-liquid. We are trying to control the function of molecular cluster in the microemulsion as an extremely small reaction space. SEM photograph shows BaSO4 nano-wire generated in the micro-emulsion. This technique is effective to produce a new functional material for our research purpose. Recently, to control supercooling of a phase change material (PCM), we are focusing on the solution structure of a supercooled aqueous solution.
|  BaSO4 nano-wire generated in the micro-emulsion |
Fundamental Study on Gas Hydrate Crystal
| Gas hydrates, which are regarded as key components in the provision for global warming problems, are one kind of inclusion components consisting of the guest molecules and hydrogen-bonded water cages. The unit lattice cell of the structure-I gas hydrate crystal is composed of two S-cages (pentagonal dodecahedron) and six M-cages (tetra-kaidecahedron having two opposite hexagonal faces and twelve pentagonal faces). We have been studying fundamental properties of gas hydrates such as thermodynamic stability, hydrate-cage occupancy by pressurization, hydrate-cage selectivity in the binary guest species and so. | 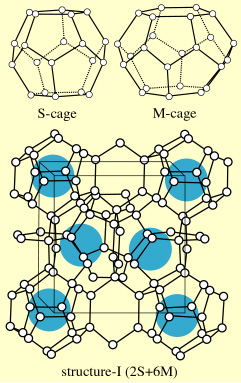 unit cell of structure-I |
Application of Gas Hydrates to Global Environmental Problems
| Gas hydrates have recently attracted many investigators' attention as key components with a counter-plan for global green-house feed back problem. Also natural-gas hydrate fields having a large amount of methane deposits have become the object of public attention as a potential natural-gas resource. We have proposed an idea of methane exploitation in linkage with carbon dioxide isolation. The methane molecule in the hydrate-cage is pushed out by the carbon dioxide molecule, and then the carbon dioxide is finally trapped in the hydrate-cage. We have also been studying carbon dioxide storage process under the deep ocean, natural-gas transport system using gas hydrate pellets, high-pressure separation of hydrogen from gas-mixtures containing carbon dioxide, hydrogen storage using gas hydrates, and temperature preservation using hydrate formation and dissociation. |