
Development of High-Performance Catalysts toward
Green Organic Syntheses
Background and Objectives of Our Research
Evolution
of environmentally-acceptable organic synthesis is an ultimate goal of the
present-day chemistry. The promising approach to “Green & Sustainable
Chemistry” is to replace conventional methods employing toxic and/or
hazardous stoichiometric reagents by atom-efficient alternatives [1]. Catalysis
is one of the leading principles to achieve high selectivity and minimization
of waste production.
The
research in the Design of High-Performance Catalysts group is directed towards
the development of highly-functionalized heterogeneous catalysts for atom-efficient,
low-waste processes for versatile intermediates for the syntheses of pharmaceutical
and agrochemicals. Especially, heterogeneous catalysts have the advantages of
being operationally simple as well as enabling unprecedented reactions based on
specific ensemble sites within a regular arrangement of surface atoms, e.g.,
metal cations and hydroxyl groups. Creating precise architectures of active
metal species on solid surfaces is one of the most important challenges in
creating highly-functionalized heterogeneous catalysts.
Current Research
Our group have developed highly-functionalized metal catalysts using unique properties of inorganic and organic materials, i.e., hydroxyapatite, montmorillonite, hydrotalcite, giant metal cluster, and dendrimer as macroligands of metal species that makes a pivotal contribution to developing environmentally-acceptable organic syntheses including selective oxidations using molecular oxygen as an oxidant and carbon-carbon bond-forming reactions.
We strive to obtain scientific insights for the catalyst and catalytic reactions in combination with application- oriented research. These goals are pursued via five research topics:
1. Development of transition metal-based heterogeneous catalytic methods for clean and selective oxidations of hydrocarbons using molecular oxygen as the sole oxidant.
2. Application of immobilized metal species in organic synthesis: development of highly selective and efficient carbon-carbon bond-forming reactions under solvent-less or aqueous conditions.
3. One-pot syntheses based on the multificntional catalysis by several active sites.
4. Preparation of nano-sized metal clusters with narrow size distribution and unique oxidation states.
5. Development of high performance nanoreactors using dendritic materials for the functional group transformations.
The following
describes the development of hydroxyapatite-bound transition metal catalysts as
a typical example.
Bone-supported
catalysts;
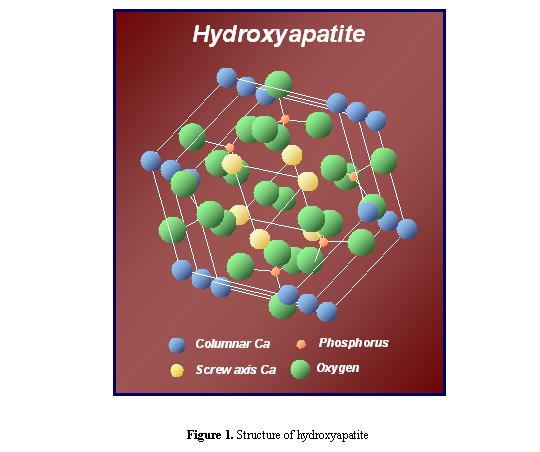
Hydroxyapatites (HAP), Ca10(PO4)6(OH)2,
possess Ca2+ sites surrounded by PO43-
tetrahedra parallel to the hexagonal axis, as shown in Figure 1 [2], and
are of considerable interest in many areas because of their ion-exchange
ability, adsorption capacity, and acid-base properties. The chemical
composition of hydroxyapatites can be varied from the stoichiometric to the
Ca-deficient form. Here, we present two new classes of hydroxyapatite- bound Pd
complexes designed with strict compositional and structural control. Both
stoichiometric and Ca-deficient hydroxyapatites are employed, and the catalysts
exhibit specific novel functions as heterogeneous Pd catalysts. The precise
construction of Pd species described here also represents a great contribution
to modern palladium chemistry.
Synthesis
and characterization of hydroxyapatite-bound palladium complexes
[3]
Hydroxyapatites
were synthesized from Ca(NO3)2×4H2O
and (NH4)2HPO4 by precipitation method.
Selecting appropriate Ca/P molar ratios gave the stoichiometric hydroxyapatite Ca10(PO4)6(OH)2
(Ca/P=1.67, HAP-0) and the Ca-deficient hydroxyapatite Ca9(HPO4)(PO4)5(OH)
(Ca/P=1.50, HAP-1). Treatment of the HAP-0 and the HAP-1 with an acetone
solution of PdCl2(PhCN)2 yielded the hydroxyapatite-bound
Pd complex, PdHAP-0 and PdHAP-1, respectively.
The characterization of the PdHAPs using physicochemical
methods such as X-ray diffraction, X-ray photoelectron spectroscopy,
energy-dispersive X-ray spectroscopy, and inductively coupled plasma (ICP)
analysis revealed that the palladium species is immobilized by adsorption on
the HAP surface. Further, the result of Pd K-edge X-ray absorption near-edge
structure spectra of both PdHAPs confirmed the divalent state of all Pd species.
A monomeric Pd species was evidenced by the absence of peaks around 2.5 A in
the Fourier transform (FT) of k3-weighted extended X-ray
absorption fine structure (EXAFS) data for the PdHAPs (Fig. 2A and 2B).
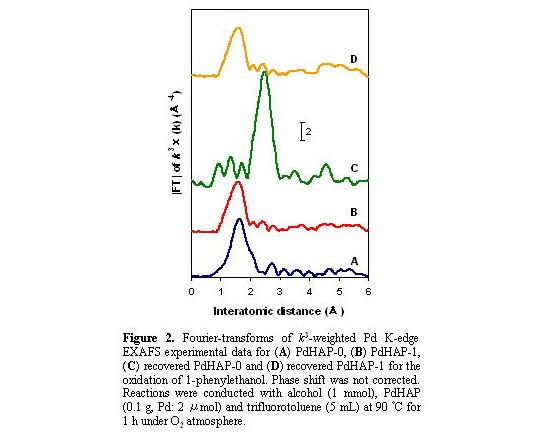
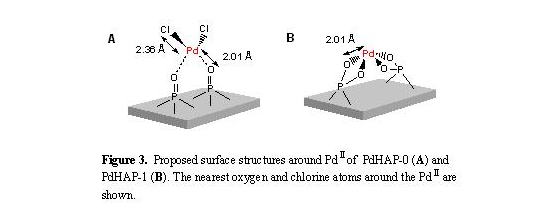
The inverse FT of the peaks around 1-2 A for the PdHAP-0 was well fitted
using Pd-Cl and Pd-O shells, whereas the best fit for the PdHAP-1 was achieved
using only a Pd-O shell. It was conclusively established that a monomeric
PdCl2
species was grafted by chemisorption on the HAP-0 surface (Fig. 3A), and
a monomeric PdII phosphate complex surrounded by four oxygens was
formed at a Ca-deficient site of the HAP-1 (Fig. 3B). Two unique
monomeric Pd species with intrinsically different surroundings can be created
on the solid surfaces through precise control of the Ca/P ratios of the parent
hydroxyapatites.
Alcohol
oxidation using molecular oxygen
The oxidation of alcohols into carbonyl compounds is
one of the most pivotal functional group transformations in organic synthesis
[4]. The PdHAP-0 proved to be an effective heterogeneous catalyst for the
aerobic oxidation of a wide variety of alcohols such as benzylic, allylic, aliphatic,
and heterocyclic alcohols, giving the corresponding ketones and aldehydes in
excellent yields. To highlight the applicability of the present protocol, a 250
mmol scale reaction of 1-phenylethanol was undertaken using 4 x 10-4
mol % of the Pd catalyst without organic solvents. The oxidation proceeded
smoothly and the turnover number (TON) of acetophenone based on Pd approached
236,000 (scheme. 1). This TON value is three orders of magnitude larger
than those previously reported for any catalyst systems under an atmospheric O2
pressure.

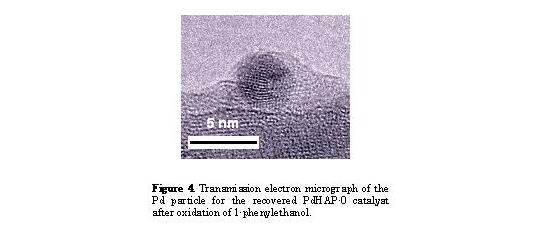
The FT EXAFS for the recovered PdHAP-0 exhibited a single peak at approximately 2.5 A due to the formation of Pd metal, as shown in Figure 2C. TEM also revealed the presence of Pd nanoparticles with a diameter of ca. 40 A having a narrow size distribution (Fig. 4). The
diameters of the generated Pd nanoparticles can be controlled upon acting on
the alcohol substrates used. Oxidation of alcohols is proposed to occur
primarily on low-coordination sites within a regular arrangement of the Pd
nanoparticles by performing calculations on the palladium crystallites [5].
ICP analysis of the filtrate confirmed that no
leaching of Pd species occurred during the above oxidations, and then the
PdHAP-0 could be reused without loss of the catalytic activity and selectivity.
It is noteworthy that the above oxidations hardly occurred in the presence of
the PdHAP-1, and that there were no structural changes around the PdII center as confirmed by EXAFS (Fig. 2D).
Carbon-carbon bond-forming reactions
The Heck coupling reaction has received considerable attention due to its
enormous synthetic potential to form new carbon-carbon bonds. Commercial
applications have, however, been limited by the low TONs and relatively
short catalyst lifetime. We found that the PdHAP-1 was an outstanding catalyst
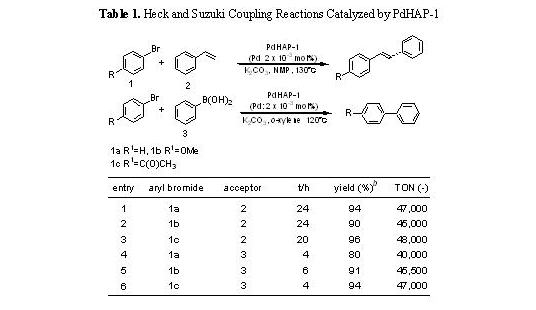
for the Heck reaction. For example, as listed in Table
1
,
the TON based on Pd reached 47,000 for 24 h in the case of bromobenzene
with styrene (entry 1). Moreover, the PdHAP-1 was applicable to the Suzuki
coupling reactions with a TON approaching 40,000 for 4 h in the reaction
between bromobenzene and phenylboronic acid (entry 4). The recovered PdHAP-1
had an original monomeric PdII structure and was recyclable with retention of its catalytic activity.
By contrast, the PdHAP-0 catalyst was less effective in the Heck reaction,
and gave a poor TON in the case of bromobenzene with styrene under the
above reaction conditions due to the formation of Pd0 particles with a diameter of ca. 50 A. It can be concluded that the high catalytic activity of the PdHAP-1 is ascribable to the exceptional robust structure of monomeric PdIIspecies under the reaction conditions.
Further
applications
Recently, we also found that the PdHAP-0 acted as an
efficient heterogeneous catalyst for the deprotection
of N-benzyloxycarbonyl group from amino acids using molecular hydrogen,
and the dehydrogenation of indolines into indoles that have served as versatile
intermediates for the synthesis of pharmaceuticals and agrochemicals [6].
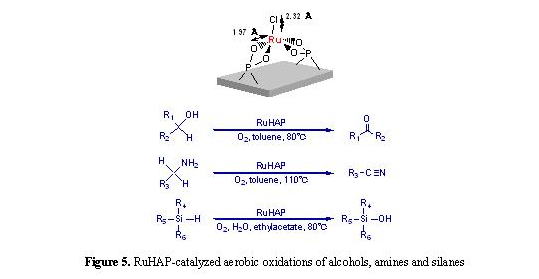
The cation-exchange ability of hydroxyapatites
enables an equimolar substitution of Ru3+ for Ca2+ on
their surface, which gives a monomeric Ru3+ phosphate species
(RuHAP) [7]. As shown in Figure 5, this RuHAP could efficiently catalyze
the oxidation of alcohols, amines, and silanes under an atmospheric
pressure of O2 [8]. Furthermore, treatment of the RuHAP with an
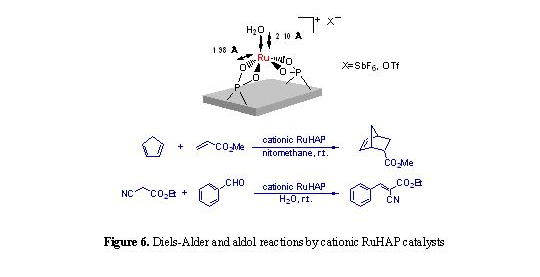
aqueous solution of AgX (X = SbF6-, TfO-)
readily afforded hydroxyapatite-bound cationic Ru complexes having potentially
vacant coordination sites [9]. Such cationic RuHAP proved to be promising
heterogeneous Lewis acid catalysts that promote Diels-Alder and aldol reactions
under mild and neutral reaction conditions (Fig. 6).
Summary
A novel approach to catalyst design of metal species using hydroxyapatite
as a macroligand and their excellent catalytic performances for aerobic
alcohol oxidation and C-C bond-forming reactions were demonstrated. These
catalytic systems can offer significant benefits in achieving simple and
clean organic syntheses since the organic syntheses described here have
following advantages; 1) easy preparations of the catalysts, 2) high catalytic
activity under mild conditions, 3) simple work-up procedures, and 4) a
reusability of harmless catalysts.
We expect that our immobilizing protocol based on the
inorganic crystals will offer an attractive route for the design of
high-performance catalysts at the atomic and molecular level, and thus have
continued to create nanostructured heterogeneous catalysts with the aim of
realizing environmentally-benign chemical processes.
References:
1)
For example,
Trost, B. M. Science 1991, 254, 1471; Sheldon, R. A. Chemtech
1994, March 38; Clark, J. H. Green Chem. 1999, 1,
1; Anastas, P. T. and Warner, J. C. Green Chemistry; Theory and Practice,
Oxford Press. 1998.
2)
Elliot, J. C. Structure
and Chemistry of the Apatites and Other Calcium Orthophosphates, Elsevier,
1994.
3)
Kaneda, K. et al.
J. Am. Chem. Soc. 2002, 124, 11572.
4)
Sheldon, R. A.; Kochi, J. K. Metal Catalyzed
Oxidations of Organic Compounds, Academic Press: New York, 1981.
5)
Kaneda, K. et al.
J. Am. Chem. Soc. In press.
6)
Kaneda, K. et al.
Tetrahedron Lett. 2003, 44, 4981; Tetrahedron Lett.
2003, 44, 6207.
7)
Kaneda, K. et al.
J. Am. Chem. Soc. 2000, 122, 7144.
8)
Kaneda, K. et al. Chem. Commun. 2001, 461;
New J. Chem. 2002, 26, 1536.
9)
Kaneda, K. et al.
J. Am. Chem. Soc. 2003, 125, 11460.